MicroMedia®
The addition of filter aids and wet strength resin differentiates MicroMedia® depth filter sheets from the AlphaMedia™ Series, but that is not where the differences end. The addition of these ingredients improves retention efficiencies by adding internal surface area and the positive charge known as Zeta-Potential. Zeta-Potential is the positively charged site within the matrix of fibers and filter aid which attracts negatively charged particles which may be smaller than the pore sizes available in the filter pad.
Wet strength resins are capable of being steam sterilized and are all free of formaldehyde. Filter aids used are perlite and/or diatomaceous earth which also help to improve filtration efficiencies.
MicroMedia® depth filter sheets are formulated with cellulose, wet strength resin, and diatomite and/or perlite is specifically designed for use in critical applications. All components of ErtelAlsop MicroMedia® depth filter sheets are listed in the CFR as generally recognized as safe for contact with food as dictated by 21CFR 176.170.
How are customers using our MicroMedia™ ?
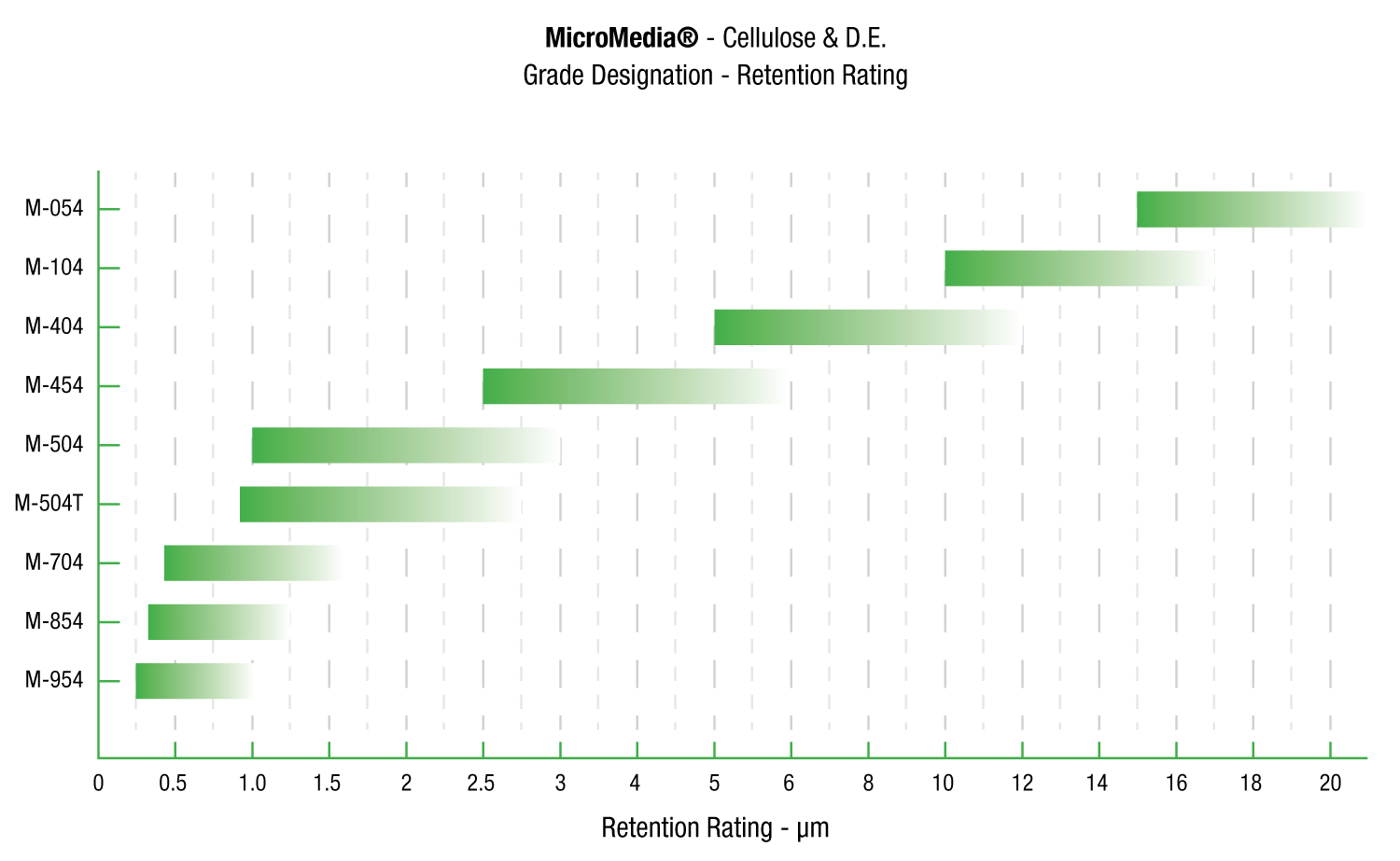
MicroMedia®
MicroMedia depth filter sheets are formulated with cellulose, wet strength resin, and diatomite and/or perlite is specifically designed for use in critical applications.
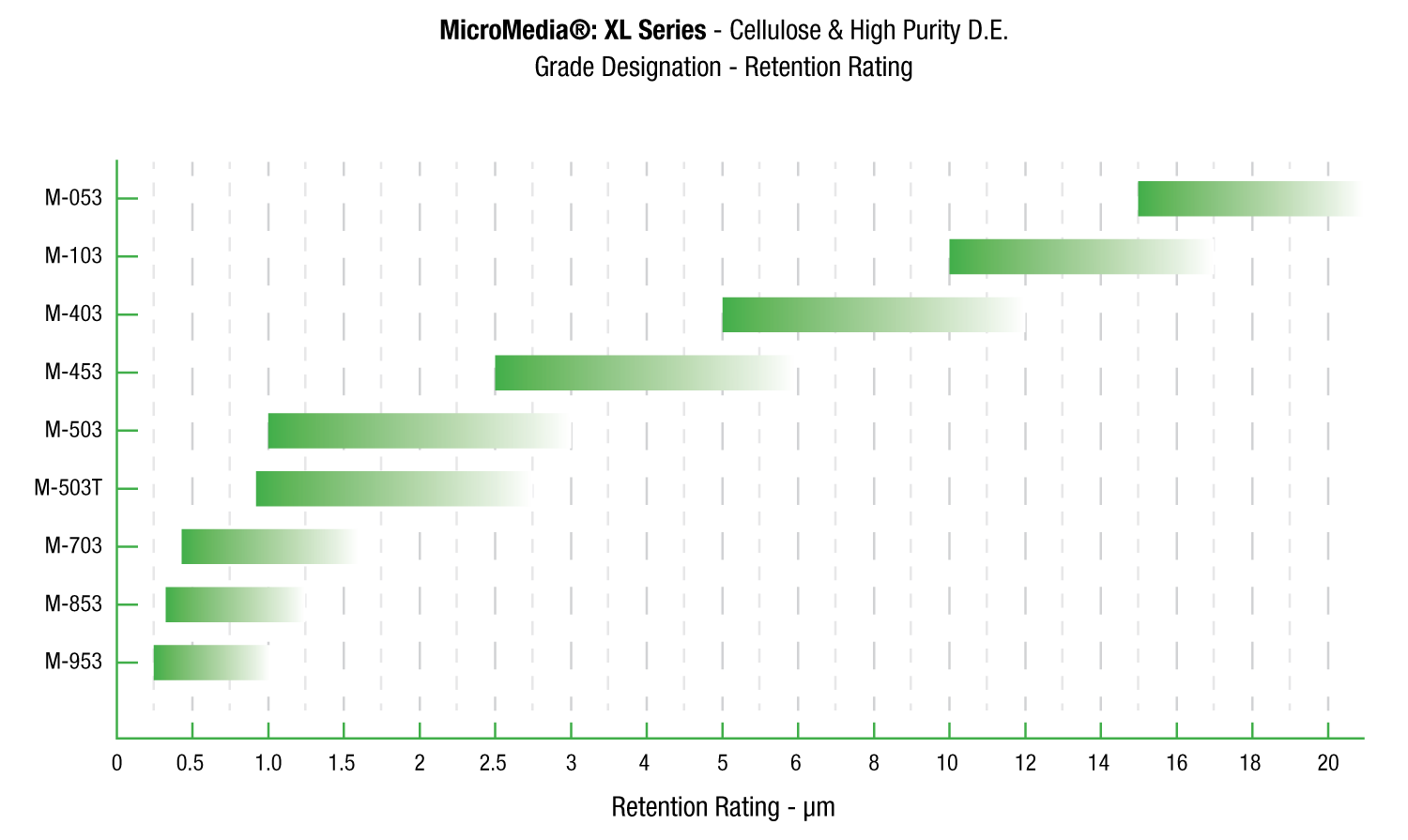
Request A QuoteMicroMedia® XL
MicroMedia® XL Series depth filter sheets are formulated with cellulose, wet strength resin and Celpure® diatomite. Celpure® is an ultra-pure form of diatomaceous earth manufactured specifically for use in critical applications.
MicroMedia® XL Series depth filter sheets provide an improved clarity and greater throughput. In independent testing, ErtelAlsop XL Series depth filter sheets outperformed competitor’s standard grades by over 500%.
Most depth filter media manufacturers currently use DE formed to “food-grade” quality standards. This allows for problems with respect to compendial standards, purity, manufacturing/process control and packaging. Unchecked variations in diatomite result in significant process deviation such as filtrate color, pH and impurity profiles. Celpure® DE can meet the high quality standards dictated by 21CFR211.160 (b). Traditional “food-grade” forms of DE rarely meet USP-NF standards.
Request A QuoteMicroMedia® LXL
For the ultimate in quality assurance, ErtelAlsop MicroMedia® LXL Series Filter Sheets are formulated with both high purity cellulose and a high quality diatomaceous earth.
The 9 grades of MicroMedia® LXL Series Filter Sheets offer the combined advantages of the XL series ultra pure Diatomaceous Earth with the L series Beta glucan poor cellulose fibers to produce this superior depth filter media and making it the leading choice for Bio-pharmaceutical applications.
Request A QuoteMicroMedia® PXL
PXL Double Layer Filter Media – Effective High Solid Filtration
Complex of Filtration Mediums
PXL media is a complex of filtration mediums that enable significantly improved throughput and superior contaminant retention in high solids loading processes. Designed specifically for applications as an initial prefilter or clarification stage filter, PXL media is ideal for protecting price and contaminant sensitive process steps further downstream.
Available Product Range/Scalable Formats
ErtelAlsop has made PXL media available in six different sizes of scalable, single-use disposable MicroCap capsules as well as 12 inch and 16 inch diameter lenticular filters for use in housings. Effective filter areas (EFA) range from as little as 23cm² to as much as 3.8 m² per 16 inch Pak lenticular cartridge.
The ErtelAlsop 12 inch diameter and 16 inch diameter lenticular filters are available in a number of different formats containing either flat gasket or locking double o-ring end fittings.
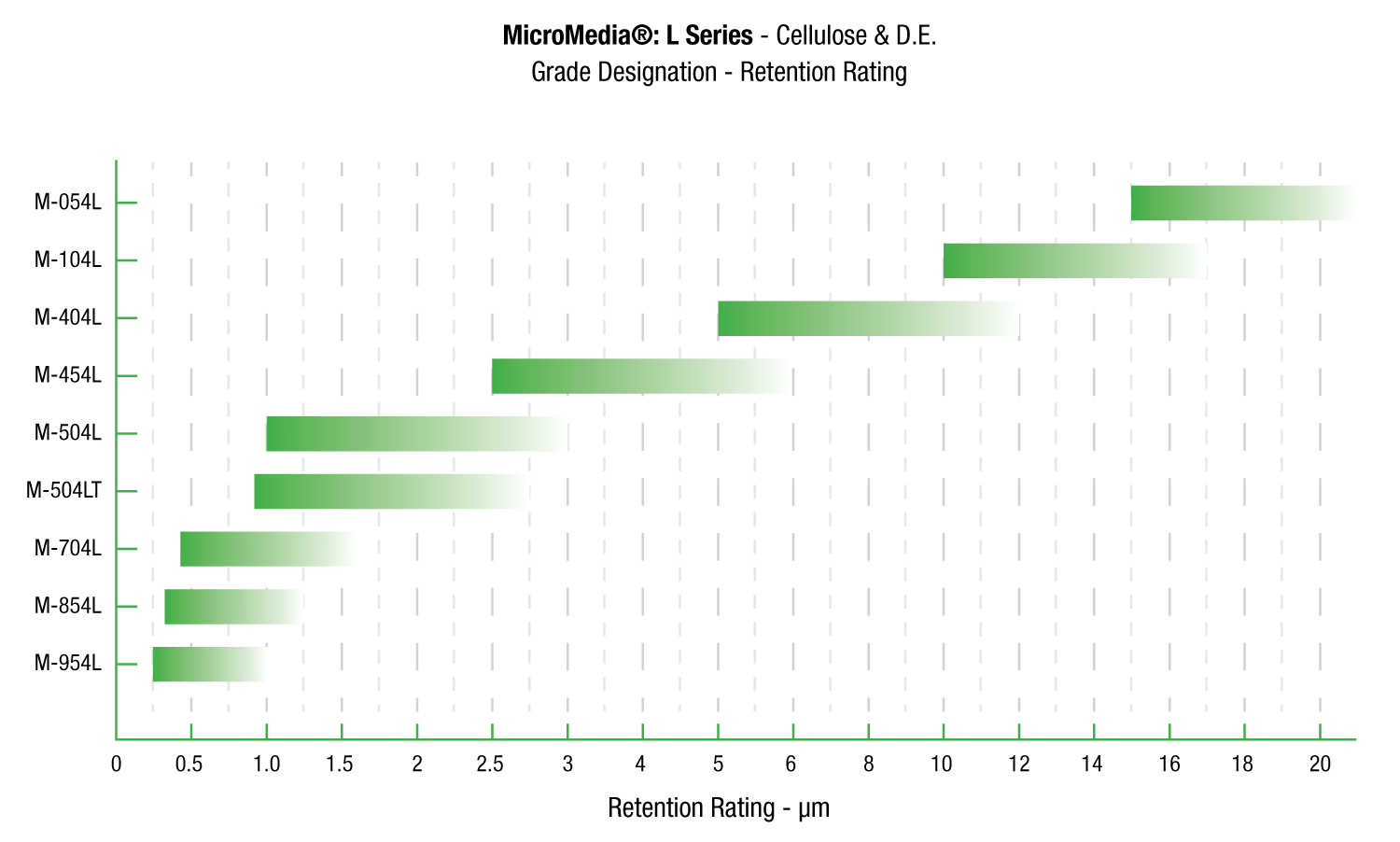
Request A QuoteMicroMedia® L
MicroMedia® L Series sheets can be formulated using high purity cellulose to reduce the occurrence of false positives caused by 1,3 beta D glucans in cellulose pulp.
MicroMedia® L Series filter sheets are specifically designed for use in applications where proteins are present, such as blood fractionation and recombinant DNA derived products. These particular types of applications demand specific controls on pyrogens and extractables. Physical attributes, unique to such applications can lower the surface tension of the media and cause them to release extractables. This can cause problems with the LAL testing used both in process and as a final release. These extractables can cause “false positives” by reacting with a Factor G in the LAL reagent and cellulose components.
A particular source of extractables is 1,3 ß D-glucan contamination in the cellulose. Cellulose is a major component of depth filter products. The level of reactivity depends upon the amount of 1,3 ß D-glucans released during manufacturing of the filter media and the amount of Factor G present in the LAL reagent, which varies by manufacturer.
Utilizing a unique process, ErtelAlsop minimizes 1,3 ß D-glucan levels in cellulose pulp prior to manufacturing the filter media. This minimizes the effects of 1,3 ß D-glucans and allows for validation of the LAL test on the product.
Request A Quote